Can Elements Have the Same Ground State Electron Configuration
Ground state electron configuration of selenium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 4. I and S B.
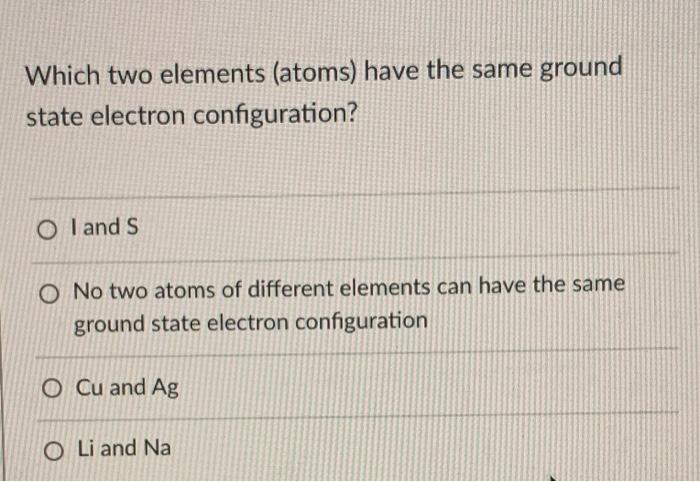
Solved Which Two Elements Atoms Have The Same Ground State Chegg Com
So the valency of iodine is 1.
. The ground-state electron configuration of the Silicon Si atom is 1s 2 2s 2 2p 6 3s 2 3p 2. In the ground state transferring of one or more electrons cannot take place in different orbitals. In the beryllium atom Z 4 the fourth electron fills the 2s orbital giving the total ground state electron configuration 1s2 2s2.
No two elements have the same ground - state electron configuration. Each sub-orbital can have a maximum of two electrons. Keep in mind electron configurations are most stable when.
For Krypton and most of the elements there are more the just one way usually two to write the electron configuration. D Cl and Ar have the same ground-state electron configuration. Ground State vs Excited State Electron Configuration Example Practice Problems Explained Summary d Cl and Ar have the same ground-state electron configuration.
Write out the electron configuration. Z 4 beryllium atom 1s2 2s2 or He2s2. The p-orbital has three sub-orbitals.
Each sub-orbital can have a. The ground state configuration of an atom is the same as its regular electron configuration in which electrons remain in the lowest possible energy. 2 1s 2s 2 3s 3p6 3d6 B.
This can be referred to as the s block the p block the d block and the f block lanthanides and actinides meaning that in its ground state an element in a certain block will have its valence electrons in the s p d or f orbitals depending. Thus all the electrons are in the lowest possible energy levels. AI and S BCu and Mg CLi and Na D.
Ground State Electron Configurations. Contains two electrons in the 1st energy level Contains four electrons in the 2nd energy level. For example The ground state configuration of the sodium atom is 1s 2 2s 2 2p 6.
How many different principal quantum numbers can be found in the ground state electron configuration of ruthenium. Then the correct electron configuration of iodineI in ground state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p x 2 5p y 2 5p z 1. This electron configuration shows that the last shell of the iodine atom has an unpaired electron.
Z 3 lithium atom 1s2 2s1 or He2s1. How many different principal quantum numbers can be found in the ground-state electron configuration of nickel. What is the electron configuration for an argon atom Ar in its ground state lowest energy state.
A 2 B 3 C 5 D 4 E 6 29. Which two elements have the same ground-state electron configuration. 2 1s2 2s 2p6 3s2 3p6 4s2 D.
2 1s 2 2s 2p6 3s2 3p6 4s 3d6 C. No 2 elements have the same ground-state electron configuration. In the selenium ground-state electron configuration four electrons of the 4p orbital are located in the 4p x 2 4p y and 4p z sub-orbitals.
Which two elements have the same ground-state electron configuration. The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration. One way is to write out the entire electron configuration by going through each orbital or we can use a shorthand notation using the noble gases as a starting point.
Your ion has a 3 charge so your electronic configuration is not the same as the ground state electron configuration. The table below shows the electron configuration for the first 20 elements on. The sub-orbitals are p x p y and p z.
The electronic configuration is the arrangement of electrons in an atomic orbital of an atom or compound. Li and Na D. A Ar 3d10 4s2 4p3 B.
Using the abbreviation He for 1s2 the configurations are. Cu and Ag C. A I and S B Cu and Ag C Li and Na D Cl and Ar E No two elements have the same ground-state electron configuration.
The ground state electron configuration of ground state gaseous neutral argon is Ne. The valency of the element is determined by electron configuration in the excited state. No two elements have the same ground-state electron configuration.
The ground-state electron configuration is calculated in the same way as deduced from the Aufbau principle. The ground state electron configuration of Fe is _____. 3p 6 and the term symbol is 1 S.
So all elements have different number of electrons with different electronic configurations. Using carbons ground state electron configuration it can be determined that carbons ground state. 119 rows The period or row numbers 1 through 7 are the energy levels of the.
All elements have different atomic number. E No two elements have the same ground-state electron configuration.

Which Two Elements Have The Same Ground State Electron Configuration How To Discuss

Solved Question 13 Which Two Elements Have The Same Chegg Com

Solved En 15 Which Two Elements Have The Same Ground State Chegg Com
No comments for "Can Elements Have the Same Ground State Electron Configuration"
Post a Comment